The biotech sector has been in the spotlight in the past week with important pipeline and regulatory updates. Vaccine updates for COVID-19 are back in focus with new authorizations for exposed populations.
Recap of the Week’s Most Important Stories
:
EUA for Moderna’s Jab for Children
:
Moderna, Inc.
MRNA
announced that the FDA has granted emergency use authorization (EUA) for its COVID-19 vaccine (mRNA-1273) for additional age groups. The vaccine has now been authorized for children aged six months through five years at a dose level of 25 µg. In addition, the EUA has been granted for a 50 μg two-dose regimen of mRNA-1273 for children six through 11 years old and a 100 μg two-dose regimen for adolescents aged 12 through 17 years. Positive interim results from the phase II/III KidCOVE study showed a robust neutralizing antibody response in the six months through five years of age group consistent with young adults, even at the lower 25 μg dose, along with a favorable safety profile consistent with other age groups.
Interim results of its phase II/III TeenCOVE study of mRNA-1273 at the 100 μg dose showed the non-inferior anti-SARS-CoV-2 neutralizing antibody responses at that dose level when compared to that in individuals 18 to 25 years old from the phase III COVE study. The antibody response was also demonstrated to be non-inferior to adults, and the vaccine efficacy in the nearly 2,500 adolescents who received the vaccine was observed to be 93% when using the CDC case definition.
ACER Down on Regulatory Update
: Shares of
Acer Therapeutics Inc
.
ACER
were down after the FDA issued a Complete Response Letter (CRL) regarding the new drug application (NDA) for ACER-001 (sodium phenylbutyrate) for oral suspension for the treatment of patients with urea cycle disorders (UCDs). The CRL indicates that the FDA cannot approve the NDA in its current form and needs to inspect a third-party contract packaging manufacturer. The regulatory body has not raised any approvability concerns related to the efficacy, safety or pharmacokinetics of ACER-001. The FDA, however, did provide one comment in the CRL requesting additional existing nonclinical information to be provided in the resubmission of the NDA. Acer has a collaboration and license agreement with its collaboration partner, RELIEF THERAPEUTICS Holding SA, for the worldwide development and commercialization of ACER-001 for the treatment of various inborn errors of metabolism, including UCDs and Maple Syrup Urine Disease (MSUD). Acer currently intends to resubmit the updated NDA for ACER-001 for oral suspension to treat patients with UCDs in the early-to-mid third quarter.
Regulatory Update From Rhythm Pharmaceuticals
:
Rhythm Pharmaceuticals, Inc.
RYTM
announced
that the FDA has approved its supplemental new drug application (sNDA) for obesity drug Imcivree (setmelanotide), a melanocortin-4 receptor (MC4R) agonist, for patients with Bardet-Biedl syndrome (BBS). The drug is now indicated for chronic weight management in adult and pediatric patients six years of age and older with monogenic or syndromic obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1) or leptin receptor (LEPR) deficiency, or BBS. However, the regulatory body issued a CRL for the sNDA for setmelanotide in Alström syndrome. Rhythm plans to re-evaluate potential paths forward in Alström syndrome in the U.S. Shares were down on the same.
Rhythm Pharma currently has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
BeiGene Up on sBLA Acceptance
: Shares of
BeiGene
BGNE
surged after the company announced that the Center for Drug Evaluation of the China National Medical Products Administration has accepted a supplemental biologics license application (sBLA) for the company’s anti-PD-1 inhibitor, tislelizumab, in combination with chemotherapy as a first-line treatment for patients with advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1. The application is supported by data from an interim analysis from the global RATIONALE 305 trial of tislelizumab versus placebo in combination with chemotherapy as a first-line treatment for these patients. This sBLA is the 10th regulatory submission for tislelizumab in China and the drug is already approved for nine indications in China. Tislelizumab is currently under review in the United States and EU for advanced or metastatic ESCC after prior chemotherapy.
Performance
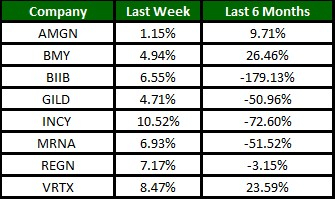
Image Source: Zacks Investment Research
The Nasdaq Biotechnology Index has gained 6.46% in the past four trading sessions. Among the biotech giants, Incyte has gained 10.52% during the period. Over the past six months, shares of Moderna have lost 51.52%. (See the last biotech stock roundup here:
Biotech Stock Roundup: BLUE Up on Regulatory Updates, RIGL’s Pipeline News & More
)
What’s Next in Biotech?
Stay tuned for more pipeline and regulatory updates.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report




